● SK Chemical Victorious in Appeals Court Patent Battle with Novartis
- Novartis’ three patent claims ruled ‘invalid’ in Appeals Court
- Case shifts in favor of SKC… Anticipated boost in domestic market
- Proof of patent ‘invalidity’ and other proactive strategies give SKC upper hand
President and Board Chairman, Insok Lee, announced on the 11th that the patent suit with Novartis over Exelon’s Alzheimer’s patch was victorious in Appeals Court.
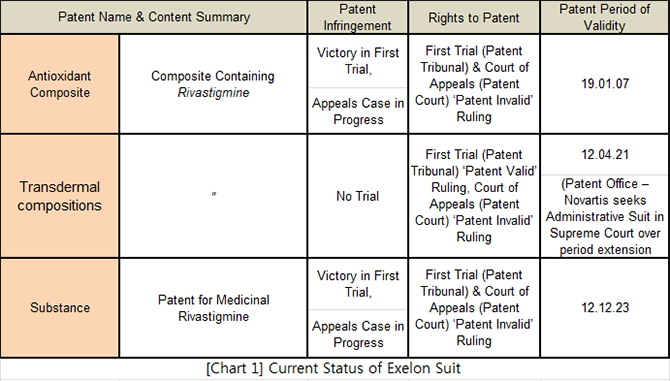
The Patents Court ruled that the two patents covering Exelon’s use of rivastigmine and the inclusion of transdermal compositions were both invalid. The ‘valid’ ruling for the transdermal compositions patent in the initial trial was only followed by the decision being overturned in Appeals Court.
The additional ‘invalid’ ruling in Appeals Court of the two patents also resulted in the three patents for the Exelon patch being ruled ‘invalid.’ Consequently, SK Chemical has obtained the higher ground in the patent suit now underway in the High Court. *Refer to Chart 1 below*
SK Chemical operations are projected to expand significantly with the September introduction of the ‘Wondron Patch’ once patent issues are resolved.
There are approximately ten generic brands of the Exelon patch sold domestically, but SK Chemical possesses the unrivaled, patent-approved ‘Wondron Patch 15.’
Head Chairman Lee announced future plans saying, “The pursuit of proving the authenticity of our product through invalidating the patent rather than simply claiming patent infringement has proven very effective. Beyond taking action on further research and development practices to increase our competitive power in the global market, we will formulate defensive countermeasures to ensure victory in the Patents Court.”
Last year in February, self-developed technology enabled SK Chemical to commence exportation of the world’s first Alzheimer’s rivastigmine treatment patch, SID710. Export destinations presently include England, Germany and about twenty other European nations plus planned expansion of SK Chemical distribution to Canada and the U.S.