● SK Chemical Revamps Label Design of Cosca Blood Pressure Medication
- Easy-to-Recognize Label
- Highly-Efficient Product Identification & Prescription Anticipated
SK Chemical announced on the 24th that the label design of Cosca blood pressure medication for both the Cosca losartan potassium supplement and Cosca complex, including Cosca Plus, Cosca Plus-Pro, Cosca Plus-F, have been newly upgraded.
SK Chemical-based product, Cosca, is classified into single supplementary 50mg / 100mg tablets and a diuretic (hydrochlorothiazide) complex medicinal, Cosca Plus. Cosca Plus is further allocated into these sub-categories--plus, plus-pro and plus-f, according to the concentrations of its two components.
There have been concerns raised over the ambiguity of distinguishing products during product circulation and prescription due to the lack of a unique package appearance for each product. SK Chemical began updating its product label designs to clearly distinguish each product by enlarging the font size of the component’s concentration and color-categorizing each product.
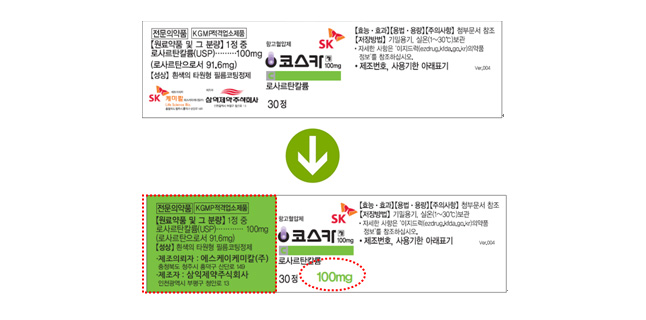
An SK Chemical official articulated that updating of product packaging and labels for products with ambiguous identifications will be conducted to promote a higher level of efficiency and convenience in product circulation and prescription.
This recent alteration in label design will not incur a product recall as only the labeling of the product was updated.
Please refer to the following for updated product labeling:
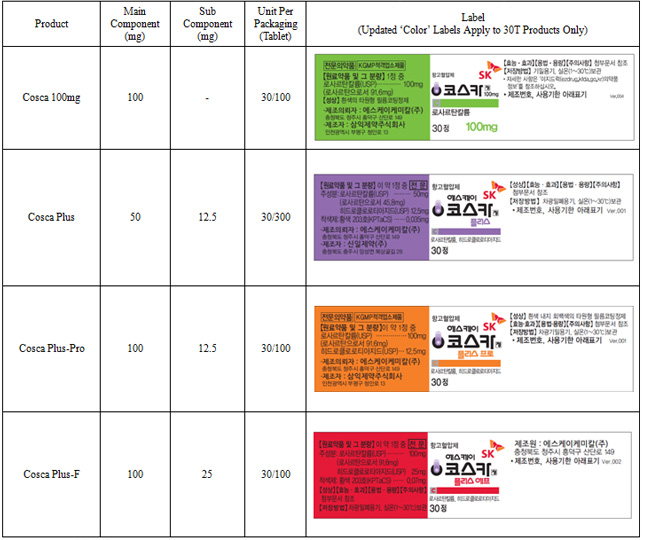
Main Component: losartan potassium
Sub Component: hydrochlorothiazide