● "AFSTYLA," a Hemophilia Therapy, Gains Approval in Canada Following Authorization in U.S.
- Approved in Canada following U.S. FDA authorization in May
- On the verge of gaining green light from EMA ∙∙∙ approval applications completed in Switzerland
and Australia
AFSTYLA, a hemophilia treatment developed by SK chemicals (President Park Man-hun), has been authorized for sale by the Canadian health authorities (Health Canada), following its approval by the U.S. Food and Drug Administration, the company announced on Dec. 18.
A bio drug developed by SK chemicals, AFSTYLA was licensed out through technology transfer to CSL in 2009. CSL has since been developing the production process, conducting clinical tests and applying for approval for sale in global markets.
For the first time as a bio-based drug developed by a Korean company, AFSTYLA entered the U.S. market in May. It is on the verge of receiving marketing approval by the European Commission for Human Medicine under the European EMA. Deliberations are also underway for marketing approval in Switzerland and Australia.
AFSTYLA is a blood coagulation factor VIII (antihemophilic factor) developed by SK chemicals for the first time in the world with a single-chain product for treatment of type A hemophilia. Unlike the existing hemophilia treatments based on the coalition of two separate proteins, AFSTYLA completely combines the two proteins, thus drastically improving their stability. It can be administered twice a week, which can also be cited as one of its prominent features.
In addition, its safety has been proved through global clinical trials; None of the patients treated with the drug reported to have showed neutralizing antibody response, which refers to the process of forming antibodies that undermine a drug`s effect. It is considered the most serious side effect of treatments for hemophilia A.
Dr. Andrew Cuthbertson, chief scientific officer and director of R & D at CSL, was quoted as saying, "Canada`s marketing authorization for AFSTYLA, a type-A hemophilia treatment, allows patients to find alternative treatments for a better life."
CSL predicts that AFSTYLA will have a competitive advantage in the global hemophilia treatment market as an innovative and effective therapy. Accordingly,, SK chemicals expects to earn royalties from CSL`s global sales of the drug.
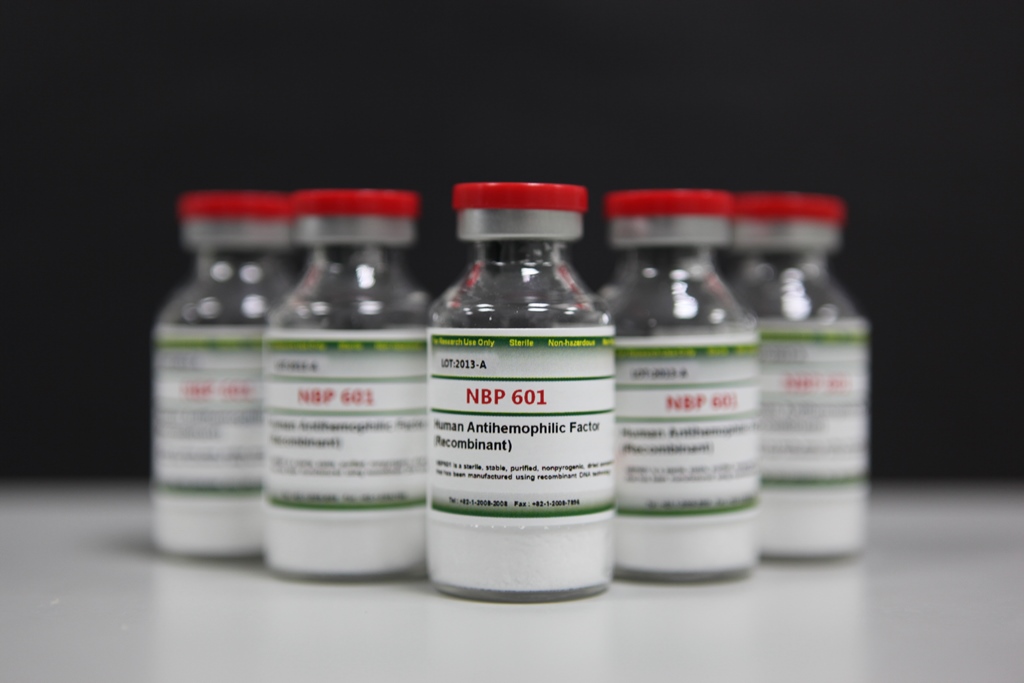
[Photo]
AFSTYLA (task name: NBP601), a treatment for hemophilia, has been approved by the Canadian health authorities.
[Terminology]
1) Hemophilia A
This refers to a congenital hemorrhagic disorder caused by deficiency of blood coagulation factor VIII. Most patients are male. In the case of patients with type A hemophilia, hemorrhage usually develops or persists in muscles, joints or internal organs. According to the Centers for Disease Control and Prevention (CDC), one in about 6,000 men is born with hemophilia.
2) AFSTYLA
This represents an abbreviation of "Affinity Style Long Acting," which means the hemophilia treatment is stable because of its strong binding force in vivo and lasts for a long time.
3) Blood Coagulation Factor VIII
There are 13 factors involved in blood coagulation. The lack of the eighth factor leads to the occurrence of type A hemophilia.
[About CSL]
CSL Limited (www.csl.com.au)
CSL founded in 1916 is a leading global biotherapeutics company with a dynamic portfolio of life-saving innovations, including those that treat hemophilia and immune deficiencies, as well as vaccines to prevent influenza. It — including its two businesses CSL Behring and Seqirus — operates in over 30 countries with more than 16,000 employees.