● SK chemicals` Hemophilia Treatment AFSTYLA Obtains Marketing Authorization in Australia
- Major Market Breakthrough Following U.S., Europe, Canada
- Royalties and Sales Milestones Expected from Sales in Global Markets
A new bio-drug, produced in partnership with SK chemicals and multinational pharmaceutical company CSL Limited, has obtained marketing authorization in Australia following the U.S. and Europe and is expanding its reach in the global market.
SK chemicals (Man-hun Park, President) announced on the 23rd of this month that AFSTYLA, a hemophilia treatment, had received final marketing authorization from Australia`s Therapeutic Goods Administration (TGA). As a result, AFSTYLA became the first domestically-developed biopharmaceutical drug to enter markets in the U.S., Europe, Canada, and now Australia.
CSL Limited has been manufacturing, conducting global clinical trials, and submitting licensing applications for AFSTYLA, developed by SK chemicals using proprietary technologies and exported to CSL in 2009.
AFSTYLA received marketing approval from the US Food and Drug Administration (FDA) in May 2016 and became the first domestic bio-drug to be sold in U.S. Furthermore, the drug subsequently obtained marketing authorization in Canada in December 2016 and Europe in January 2017. The drug is currently in the approval screening stage in Switzerland and Japan.
AFSTYLA is an antihemophilic factor (AHF), or Factor VIII (FVIII), and world`s first single-chain product for hemophilia A. While conventional hemophilia treatments have been a combination of two separate proteins, AFSTYLA completely combines two proteins for improved stability, and it allows for continuous hemophilia management with just a twice-per-week dosing.
CSL Limited expects AFSTYLA to be positioned as an innovative treatment option in the global hemophilia treatment market, and accordingly, SK chemicals expects royalties and sales milestones based on global sales.
Andrew Cuthbertson, Chief Scientific Officer and R&D Director of CSL, stated, "Patients will have a treatment alternative for a better life through the recent marketing authorization of AFSTYLA in Australia." Gavin Finkelstein, President of the Haemophilia Foundation Australia, said, "The community is very pleased to see the new treatment opportunities for people with haemophilia A."
SK chemicals President Man-hun Park said, "The results of our long-term investments in the vaccine and blood product bio business are proving our capabilities in the global market." He added, "We will continue to invest in advancing the health of the world’s populace."
According to a 2015 report by the World Federation of Hemophilia, about 146 million IUs (international units) of FVIII-deficiency hemophilia A treatments are prescribed in Australia each year. Of these, 95% are recombinant products such as AFSTYLA. Currently, the average price of recombinant FVIII hemophilia treatments is around $ 3 to $ 6 AUD for 10 IUs.
According to global research firm Datamonitor Healthcare (www.datamonitor.com), the global market for hemophilia A treatments is about ₩8.2 trillion ($7.21 billion USD) annually. Related markets are growing steadily, and by 2020, the global market is expected to increase by about 17% to ₩9.5 trillion ($8.32 billion USD).
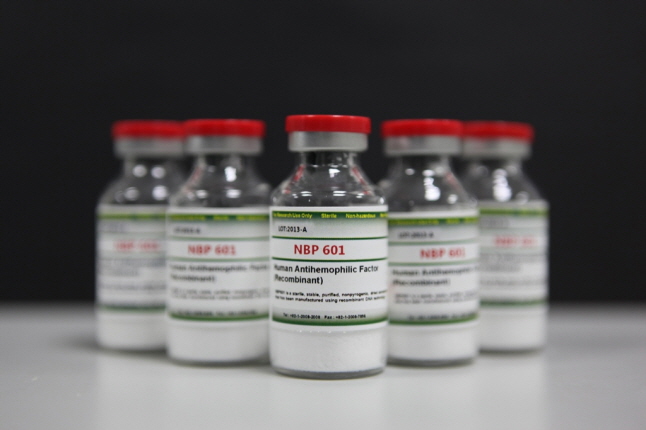
[Photo]
AFSTYLA, developed by SK chemicals and licensed to CSL Limited, obtained marketing authorization in Australia following the U.S., Europe, and Canada.
[Glossary]
* Hemophilia A
Mostly affecting males, hemophilia A is a congenital hemorrhagic disorder caused by a deficiency of blood coagulation factor VIII. Bleeding mainly occurs or persists in muscles, joints, and internal organs in hemophilia A patients. According to the U.S. Centers for Disease Control and Prevention (CDC), about 1 in 6,000 men born are born with hemophilia.
* AFSTYLA
As a portmanteau of `Affinity Style Long Acting,` AFSTYLA is a hemophilia treatment with high bond strength and stable and long-lasting effects.
* Factor VIII
Factor VIII (FVIII) is the eighth of thirteen factors involved in coagulation. Hemophilia A occurs in patients deficient in FVIII
[Company Information]
* CSL Limited (www.csl.com.au)
Founded in 1916, CSL Limited is a global biopharmaceutical company with a diverse portfolio of products including hemophilia and immunodeficiency diseases treatments as well as influenza vaccines. CSL (including CSL Behring and Seqirus) employs over 16,000 employees in more than 30 countries.