● Efficacy, Safety of New Combination OAB Drug Validated at KUA Conference
- Clinical Results Announced to an Assembly of 1,500 Urological Specialists
- "Efficacy and safety validated" "Side effects of existing drugs improved"
A consensus has been reached on the efficacy and safety of a new overactive bladder (OAB) treatment at at prestigious international urological conference.
SK chemicals recently announced the clinical results of their new combination OAB drug THVD-201 at the 69th Annual Meeting of the Korean Urological Association, held over three days at The-K Hotel in Seoul from the 27th of this month.
This annual conference is South Korea`s most authoritative integrated conference where urological specialists throughout the country gather to share the latest insights.
At this year`s conference, SK chemicals announced the efficacy and safety of THVD-201, which completed clinical trials last year.
THVD-201 is a new combination OAB drug that improves a side effect of existing antimuscarinic drugs that caused xerostomia (dry mouth) in patients. Paradoxically, conventional OAB treatments caused dry mouth, leading to increased water intake and thus urine volume in patients suffering from urinary incontinence. The new drug was developed to simultaneously treat OAB and xerostomia.
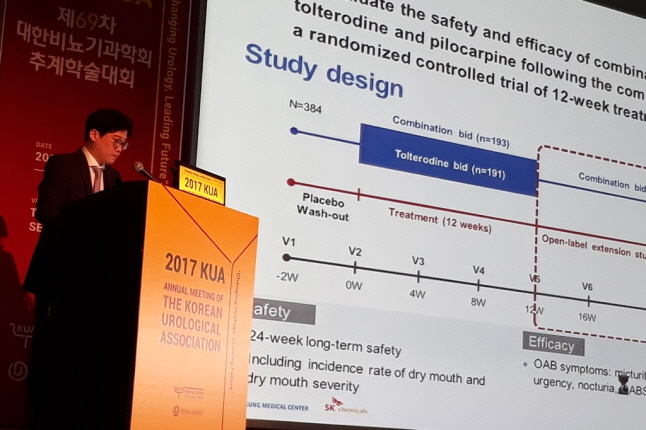
A total of 386 patients suffering from OAB participated in Phase III clinical trials conducted over 21 months since 2015 by 16 domestic medical institutions including Samsung Medical Center, proving the efficacy of the drug in controlling OAB while minimizing the xerostomia side effect.
Kwang-jin Ko, a urologist from Samsung Medical Center who presented at the conference, stated, "As evidenced by the Phase III clinical trial results, a 12-week treatment with THVD-201 effectively reduced symptoms of xerostomia while maintaining efficacy." He added, "Continued efficacy and safety was observed even with long-term use over a 24-week treatment period."
THVD-201`s clinical results were also announced at ICS 2017 in Florence, Italy earlier this month, attracting attention from an international society of professionals.
The medical professionals gathered at this year`s conference showed a high level of interest in Ko`s presentation, and lively discussions on the efficacy and safety of THVD-201 ensued.
Recently, the industry has seen a rise in urological departments and societies conducting independent academic activities, and about 1,500 members participated in this year`s annual KUA conference.
[Photo]
Urologist Kwang-jin Ko of Samsung Medical Center presenting at the 69th Annual Meeting of the Korean Urological Association