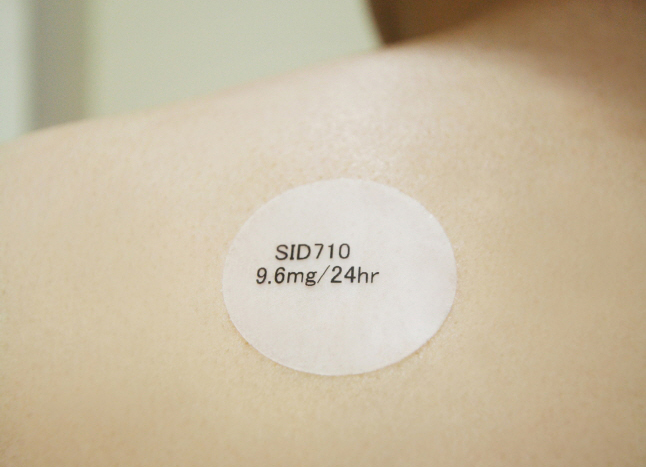
● SK chemicals Challenges US Dementia Patch Market, Files for Approval with FDA to Sell SID710
- SK chemicals filed for FDA approval on April 1 (Korean time) FDA. SID710`s is expected to launch in 2018.
- Propelled by its No. 1 position in the EU generic market, SK Chemcials is aiming at the US market.
SK chemicals’ patch-type dementia treatment is entering the US market.
SK chemicals (CEO Park Mahn-hoon) announced on April 1 (March 31 in the US) that it filed for approval with the USFDA (Food and Drug Administration) to sell its patch-type dementia treatment SID710.
In 2013, the company received approval for SID710 in the EU as the EU`s first Exelon patch generic and launched the product in 13 major European countries, including Germany, France, the U.K. and Spain. SID710 now accounts for about half of the generic market in the generic market. It has been ranked No. 1 in market share for three years in a row.
The U.S. patch-type dementia drug market is valued at $618 million (KRW712.4 billion) according to 2015 IMS data.
“We are capitalizing on our technology to expand our presence in an advanced overseas market,” said Park Mahn-hoon, CEO of SK chemicals. “We will never be satisfied and will grow into a global enterprise by exporting various product groups, including vaccines and blood derivatives.”
SK chemicals has led the development of various new drugs and is rewriting the history of new drug development in Korea: △ Sunpla, the first new drug in Korea △ JOINS, the first natural drug in Korea △ MVIX S, the world’s first film-type PDE5 inhibitor for erectile dysfunction and △ SKYCellflu, the first cell-cultured influenza vaccine in Korea.
[Photo]
SK chemicals SID710